|
|
| Name
| Condensed Structural Formula
| Structure
| Ethyne
| HC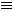 CH CH
| 2 carbon atoms
| Propyne
| HC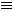 CCH3 CCH3
| 3 carbon atoms
| 1-butyne or but-1-yne
| HC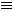 CCH2CH3 CCH2CH3
| Triple bond begins at carbon 1
| 2-butyne or but-2-yne
| CH3C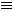 CCH3 CCH3
| Triple bond begins at carbon 1
| | | | |
Structural diagrams of complex alkynes shown in the "Examples" section:
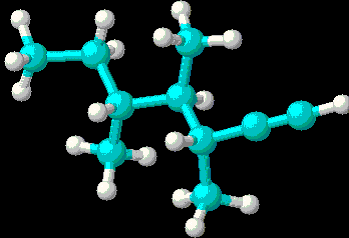
- 3,4,5-trimethyl-1-heptyne:
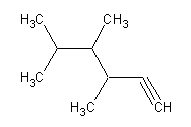
- 3,4,5-trimethyl-1-heptyne: (3D Structure)
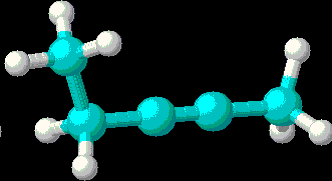
- 2-pentyne: (3D Structure)
Note: Condensed structural formula just means the structural formula showing the carbon bonding and hydrocarbon groups. The example used for nomenclature (in your brochure) is a condensed structual formula. Also used in Question #1 of Self Evaluation.
To write the chemical formula for an alkyne, given it's condensed structural formula, simply count the number of carbons and the number of hydrogens. Then write C and H and write the counted numbers as the respective subscripts.
|
|