|
|
|
Back 
| ||||||||||||||||||||||||||||||||||||||
| Answers
| ||||||||||||||||||||||||||||||||||||||
| 1. | a. | 2-hexene : Not an alkyne. Because it ends with "ene" and alkynes should end with "yne". |
| b. | C5H10 : Not an alkyne. Because it does not follow the rule of CnH2n-2. | |
| c. | C7H12 : This is an Alkyne. | |
| d. | 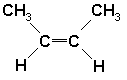 : Not an alkyne. Because it has a double bond while alkynes should have triple bonds. : Not an alkyne. Because it has a double bond while alkynes should have triple bonds.
| |
| 2. | a. | 2-butyne : C4H6
Explanation: - There is only one chain, so it is the longest. It has 4 carbon atoms, so by the prefix rules, it is butyne. - The carbon triple bond starts on the second carbon atom, so we use 2-butyne. - And there are no substituents (branches). |
| b. | 4,4-dimethyl-2-pentyne : C7H12
Explanation: - The longest chain has 5 carbon atoms. So, by the prefix rules, it is pentyne. - The carbon triple bond starts on the second carbon atom from the left. So it is 2-pentyne. - There are two methyl groups attached to carbon 4. So that will be 4,4-dimethyl. | |
| c. | 2-pentyne : C5H8
Explanation: - There is only one chain and it has 5 carbon atoms. So, by the prefix rules, it is pentyne. - The carbon triple bond starts on the second carbon atom from the right. So it is 2-pentyne. - And there are no substituents. | |
| 3. | a. | 1-heptyne
Start out with the longest chain which is heptyne. And it is 1-heptyne. So there are 7 carbon atoms and the carbon-carbon triple bond begins at the first carbon atom.
There are no substituents. Now draw all the hydrogen atoms needed for all the carbon atoms to fulfill their octet rule.
Now condense the diagram. It will look like this:
|
| b. | 2,3-dimethyl-4-octyne
Start out with the longest chain which is octyne. And it is 4-octyne. So there are 8 carbon atoms and the triple bond begins at the fourth carbon atom.
There are methyl groups on the second and the third carbon atoms. Now draw all the hydrogen atoms needed for all the carbon atoms to fulfill their octet rule.
Now condense the diagram. It will look like this:
| |
| c. | 3,4-dimethyl-1-pentyne
Start out with the longest chain which is pentyne. And it is 1-pentyne. So there are 5 carbon atoms and the triple bond begins at the first carbon atom.
There are methyl groups on the third and the fourth carbon atoms. Now draw all the hydrogen atoms needed for all the carbon atoms to fulfill their octet rule.
Now condense the diagram. It will look like this:
| |
| d. | 2,2,5,5-tetramethyl-3-hexyne
Start out with the longest chain which is hexyne. And it is 3-hexyne. So there are 6 carbon atoms and the triple bond begins at the third carbon atom.
There are 2 methyl groups each on the second and the fifth carbon atoms. Now draw all the hydrogen atoms needed for all the carbon atoms to fulfill their octet rule.
Now condense the diagram. It will look like this:
| |
| 4. | No, it is not possible to have a compound called 4-hexyne.
Hexyne has a chain of 6 carbon atoms. In 4-hexyne, the triple bond has to start at carbon 4. If the triple bond starts at carbon 4, you numbered it from the wrong side. Number it from the side that the triple bond is closer to, and you will see that it actually starts at the first carbon from the other side, so it will be 1-hexyne. | |
| 5. | a. | C3H4 : Propyne
- First make sure it is a propyne by checking whether it follows the CnH2n-2 rule. - By the prefix rules, if there are 3 carbon atoms, use the prefix "prop-" |
| b. | C5H8 : Pentyne
- First make sure it is a propyne by checking whether it follows the CnH2n-2 rule. - By the prefix rules, if there are 5 carbon atoms, use the prefix "pent-" | |
| ^ Back to Top ^ | ||